To assist our understanding of the diverse structural characteristics of sour corrosion products, the
interactive crystal structure visualization software package CrystalMaker 1.4.4 was recently purchased. Lattices are generated from inputted
structural data, usually as “Crystal Information Files” (CIFs) from online databases. CIFs are a standard method of reporting crystallographic
data for individual structures, i.e., unit cell information, atomic coordinates and space group. The benefit of using CrystalMaker is that
generated crystal structures can be manipulated, coordination environments displayed and basic measurements performed (bond lengths/angles,
density). The software is user friendly and enables generation of crystal structure images for use in reports, papers, presentations,
proposals, etc. Most importantly, CrystalMaker gives the researcher a “feel” for the structures with which they are working; this has proved
useful for visualizing, and helping develop an understanding of, corrosion products such as siderite (FeCO3), mackinawite (FeS),
troilite (FeS), pyrrhotite (Fe1-xS), smythite (Fe3+xS4), greigite (Fe3S4) and pyrite
(FeS2). Mackinawite and troilite/pyrrhotites are routinely observed in sour corrosion systems, as well as in our on-going research
at the ICMT. Stoichiometric versions of each phase would have the formula FeS, with a pyrrhotite that is not deficient in iron possessing the
troilite lattice. Structurally, the lattices of mackinawite and troilite are completely different. Each phase tends to form under particular
conditions.
Mackinawite is a layered iron sulfide, often written with the formula Fe1+xS as it is usually
found with a non-stoichiometric composition. It is readily formed at temperatures of less than 100°C at relatively short times in sour corrosion
systems. Mackinawite has a tetragonal unit cell: a = b = 3.6735 Å, c = 5.0328 Å, c = 5.0328 Å; α = β = γ = 90°.
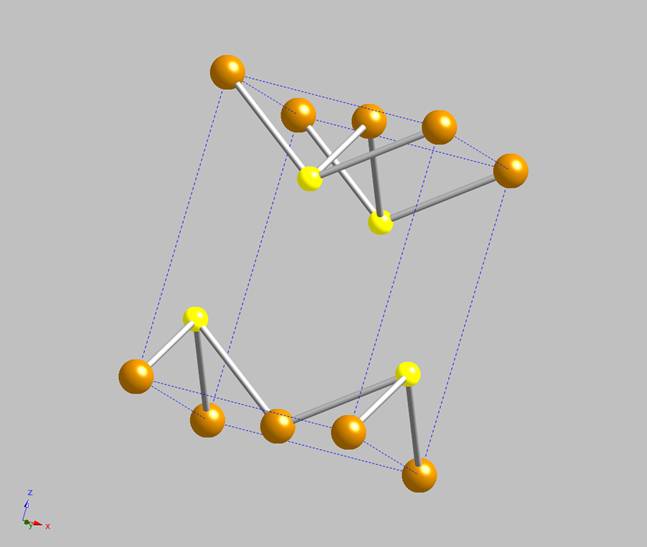
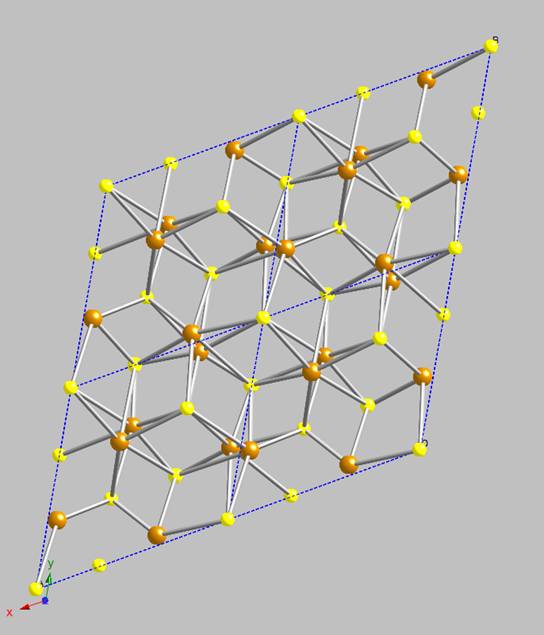
Consequently, it is frequently referred to as “tetragonal iron sulfide”. A single unit cell is shown in
Figure 1 (brown = iron, yellow = sulfur). The iron atoms are at the apices and at
the center of the square faces on opposite sides of the unit cell. The sulfurs are positioned on the elongated sides of the unit cell. The long
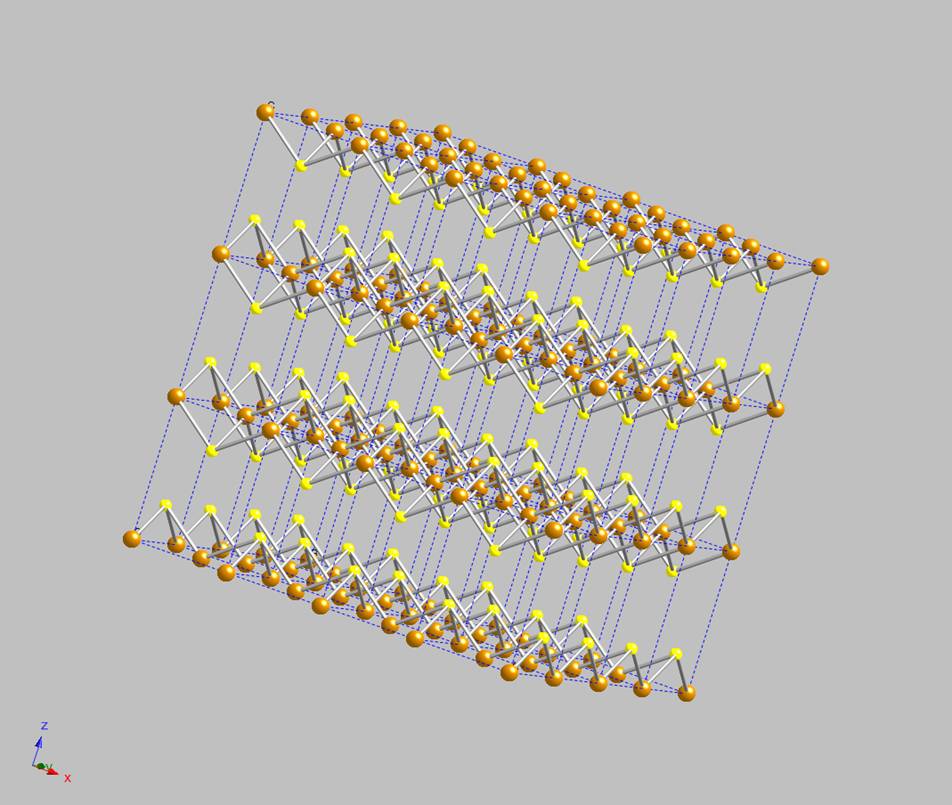
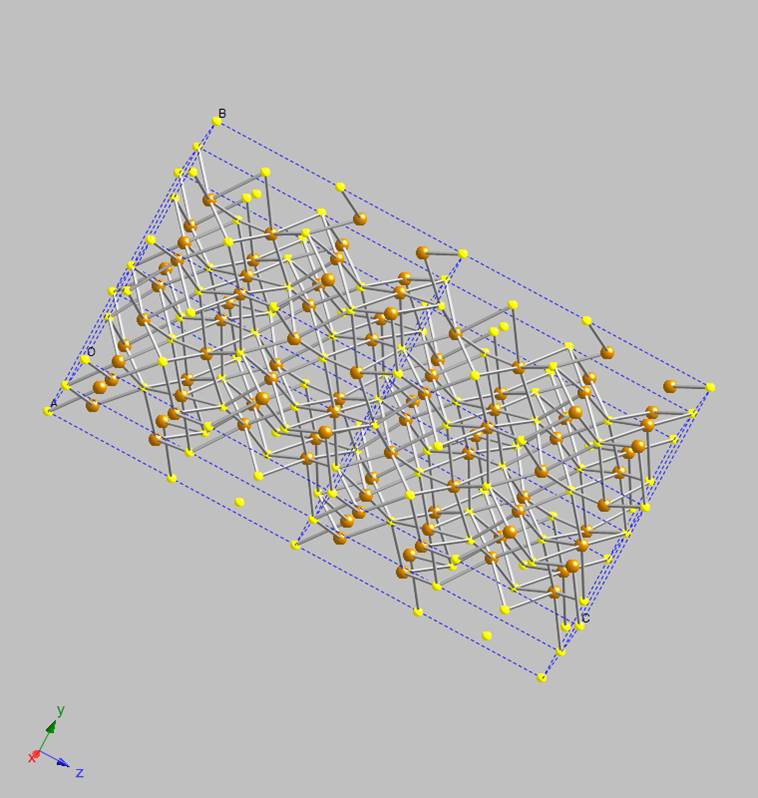
axis of the cell corresponds to the direction in which the iron sulfide layers within the mackinawite structure stack, multiple unit cells are
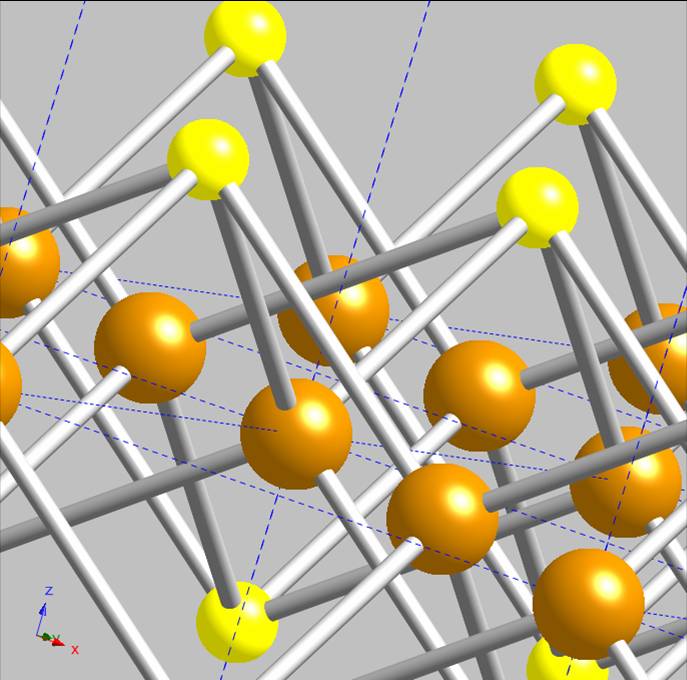
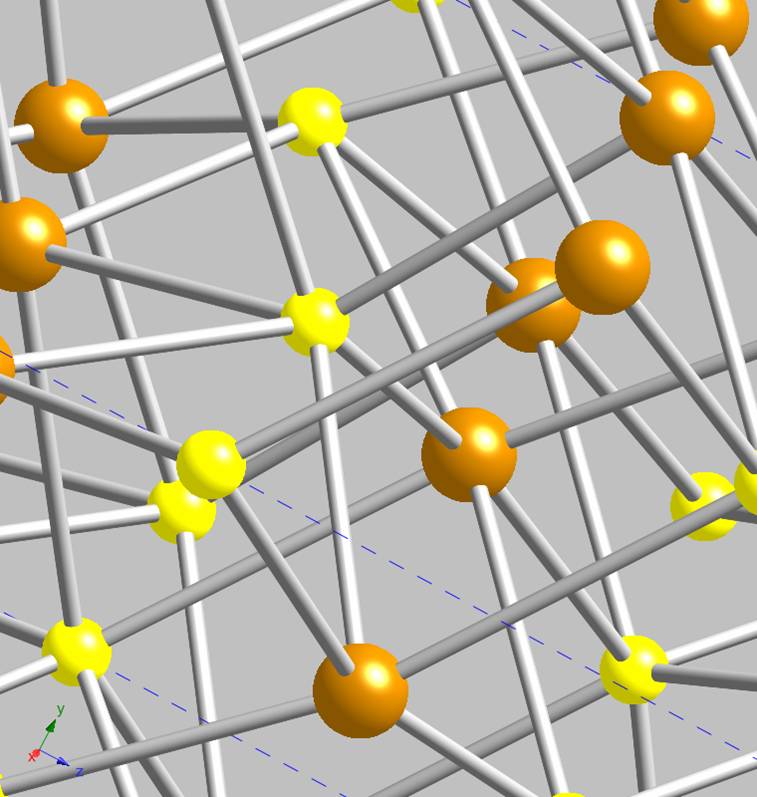
shown in Figure 2; its 2-dimensional structure is now obvious. A close-up of the
crystal structure reveals that the iron atoms are essentially embedded within each layer, tetrahedrally coordinated to sulfurs. The sulfurs are
similarly 4-coordinate, but in more of a pyramidal geometry, Figure 3. Mackinawite
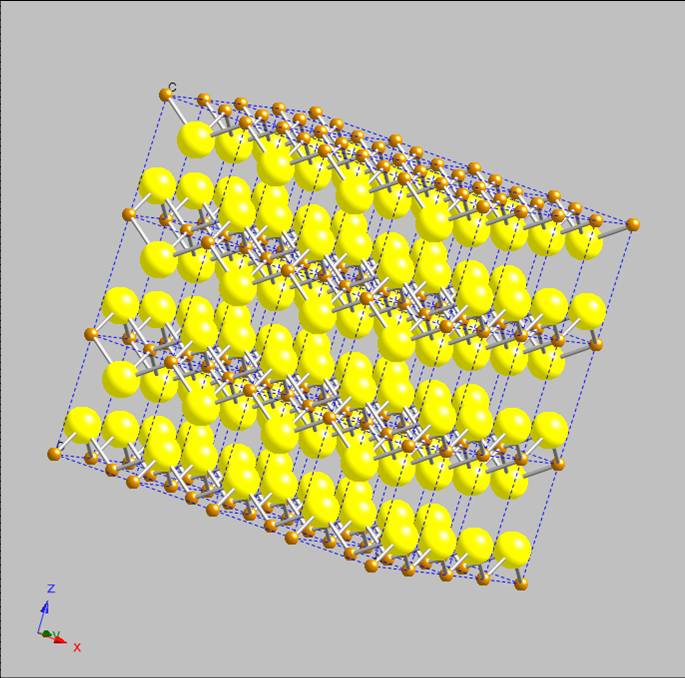
layers depicted with Shannon radii (Fe2+ radius set to 0.63 Å, S2- to 1.84 Å), which more accurately reflect the
actual/relative sizes of the atoms in the lattice, see Figure 4, reveal that the
layers are sufficiently far apart for species to reside/diffuse “between the sheets”. Wolthers et al. have hypothesized that water molecules
intercalate into the mackinawite lattice during its hydrothermal formation.
Troilite and the pyrrhotites essentially possess the niccolite, or nickel arsenide, characteristic crystal
structure; albeit with distortions and, in the case of pyrrhotites, iron deficiency. In corrosion systems, these phases typically occur at
temperatures in excess of 100°C, although their formation over long periods of time at lower temperatures may also be possible. The hexagonal
arrangement of iron and sulfur atoms in the troilite lattice, viewed perpendicular to the xy-plane, is shown in
Figure 5. Troilite can be described as an expanded/distorted hcp anion array with
cations in the octahedral holes, or vice versa. Its unit cell parameters are a = b = 5.9650 Å, c = 11.7570 Å; α = β = 90°,
γ = 120°. Unlike mackinawite, troilite possesses a 3-dimensional framework. It is worth noting that troilite is of significantly higher
density than mackinawite, 4.835 g/cm3 versus 4.298g/cm3.
Figure 6 depicts a view of its lattice after it has undergone rotation to illustrate
the alternating nature of the iron and sulfur within the crystal structure. Inspection of the figure reveals the stoichiometric nature of
troilite, with Fe:S = 1:1. The 6-coordinate, distorted octahedral character of the irons and sulfurs within the lattice is clear from
Figure 7. Pyrrhotite has essentially the same framework architecture as troilite,
except that irons are systematically absent from the lattice. Pyrrhotite formulae tend to be written as Fe1-xS, where x < 0.20,
or FeySz. The non-stoichiometry results from replacement of Fe2+ in the lattice with Fe3+, fewer of
which are required for charge balance of the S2- anions.
A long term goal of the CC-JIP is to study the formation and interconversion of iron sulfides under
conditions where localized corrosion occurs.
References
(1) Lennie, A.R.; Redfern, S. A. T.; Schofield, P.F.; Vaughan, D.J. Synthesis and Rietveld Crystal Structure Refinement of Mackinawite,
Tetragonal FeS. Mineralogical Magazine 1995, 59, 677-683.
(2) Wolthers, M.; Van der Gaast, S.J., Rickard, D. The Structure of Disordered Mackinawite, American Mineralogist, 2003, 88(11), 2007-2015.
(3) Skala, R.; Cisarova, I.; Drabek, M. Inversion Twinning in Troilite. American Mineralogist, 2006, 91, 917-921.
To download a pdf version of this click here.




